In other words a nucleus of a radionuclide has no memory. Radioactive decay occurs when an unstable atomic nucleus emits particles or light waves.

Radioactivity And The Types Of Radioactive Decay
Radioactive atoms are inherently unstable.

. It is the process that causes a huge amount of energy to be released into the surroundings in the form of ionizing radiation. This can mean the nucleus has either too many protons or too many neutrons. Radioactive decay is a random process by which unstable atoms with an excess of particles andor energy emit radiation to achieve stability.
10 Why are some substances radioactive. Learn about the different types of. Occurs in nucleus which is protons and neutrons.
When the atoms of an element have extra neutrons or protons it creates extra energy in the nucleus and causes the atom to become unbalanced or unstable. The unstable nucleus of radioactive atoms emit radiation. 6 How are radioactive elements formed.
Many atoms are unstable even if they occur in nature. Seldom happens on its own. Join with atoms of another element C.
Isotopes are atoms of the same element thereby having the same number of protons which differ in the number of neutrons in their nucleus. This is called radioactive decay. 9 What causes radioactive decay.
The decay constant λ which is the same as a rate constant discussed in the kinetics chapter. Non-radioactive elements are stable and wont commence into radioactive decay. Occurs when elements atomic is greater than 83.
Radioactivity occurs when an unstable atom emits radiation to achieve stability. Radioactive decay occurs when atoms of an unstable element. Because gamma radiation is a wave and not a particle it does not change the composition of the nucleus.
When this occurs a new atom and element are formed. So you are thinking if only one atom of an unstable isotope were somehow to be isolated in a vacuum chamber it would no longer have. Isotopes are atoms of the same element thereby having the same number of protons which differ in the number of neutrons in their nucleus.
Radioactive decay is a random process at the level of single atoms in that according to quantum theory it is impossible to predict when a particular atom will decay. Answer 1 of 11. - atomic number and atomic mass remain the same therefore the atoms identify does not change after this emission.
Break down to form atoms of another element D. Eg a neutron is converted to a proton or particles are emitted and then energy is released as gamma rays or x rays. Learn more about radioactive decay by modeling the process using coin flips.
Three of the most common types of decay are alpha decay α-decay beta decay β-decay and gamma decay γ-decay all of whi. To be more precise if the number of neutrons surpasses the number of protons the element becomes unstable because atoms tend to expel excess energy to regain stability. If the rate is stated in nuclear decays per second we refer to it as the activity of the radioactive sample.
Radioactive decay also known as nuclear decay radioactivity radioactive disintegration or nuclear disintegration is the process by which an unstable atomic nucleus loses energy by radiation. Facebook Twitter Pinterest Instagram. Radioactive decay occurs when an unstable atomic nucleus loses energy by emitting energy in the form of emitted particles or electromagnetic waves called radiation.
Are exposed to chemical weathering. Decay rate λN with λ the decay constant for the particular radioisotope. Unstable isotopes with three kinds of energy particles which means.
100g of radioactive element X are placed in a sealed box. The rate for radioactive decay is. Radioactive decay means the presence of an unstable atom.
Whether radioactive elements can become stable and if so how. Grateful dead sweatshirt vintage. Basically there is too much energy inside the nucleus to hold all the nucleons together.
A material containing unstable nuclei is considered radioactive. In the case of radioactive decay instability occurs when there is an imbalance in the number of protons and neutrons in the atomic nucleus. This could then be incorporated into other data.
The half-life of this isotope of X is 2 days. If a sample of C-14 initially contains 11 mmol of C-14 how many millimoles will be. Isotopes are atoms of the same element.
An atom means its not a vacuum because the word vacuum means the absence of atoms. The half-life for the radioactive decay of C-14 is 5730 years. Radioactive Decay When elements have unstable nuclei they decay forming more stable nuclei and.
Nuclear decay Radioactive decay occurs when an unstable atom loses energy by emitting ionizing radiation. Radioactive decay occurs when an unstable atomic nucleus loses energy by emitting energy in the form of emitted particles or electromagnetic waves called radiation. Radioactive Decay some atoms are unstable occasionally.
It results in parent elements to TRANSMUTE daughter elements which is alpha beta gamma. Radioactive decay occurs when atoms of bartleby. 7 Which is the main source of nuclear radiation.
Some isotopes of a given element are more unstable than others causing a nuclear reaction which releases energy to achieve a more. Radioactive decay is the process by which an unstable atomic nucleus loses its energy by emitting radiation. How long will it take for 25 of the C-14 atoms in a sample of C-14 to decay.
It is a by-product of alpha and beta radiation. Over time radioactive parent atoms decay into stable daughter atoms. A radioactive decay generally occurs in unbalanced atoms of certain radioactive elements.
Therefore a particle transformation takes place. 8 Where does nuclear radiation occur. 5 Why do nuclei become unstable.
An excess of neutrons and protons can cause this instability which leads to the emission of alpha particles beta particles or high-energy photons gamma radiation. 31 Why are some atoms radioactive quizlet. It is important to have a thorough knowledge of all the three rays i.
Typically occurs during the decay of an atom. Become part of a fossil B. Gamma decay results in the release of gamma radiation from the nucleus of an unstable atom.
Every atom seeks to be as stable as possible. 00g sample of As-81 to decay to 6. The status of the electrons of an atom doesnt matter for decay although they too have their.
The ionized radiations may include alpha particles beta. Radioactive elements are unstable and will decay into other elements in a decay chain. - a form of electromagnetic energy can be stopped with thick 15cm lead or concrete.

What Causes Radioactivity Decay Disintegration Unstable Nucleus Experiment To Separate Three Types Of Atomic Radiation Ks4 Science Igcse Gcse Gce A Level Chemistry Physics Revision Notes
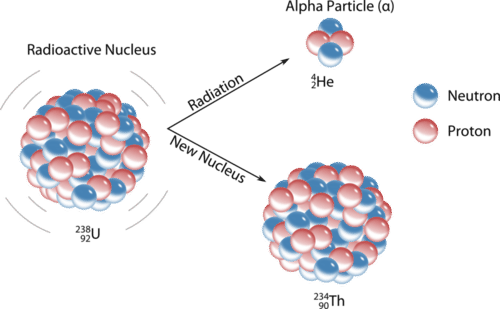
2 5 Nuclear Decay Chemistry Libretexts

Solved Radioactive Decay Occurs When Atoms Of An Unstable Chegg Com

0 Comments